Single ventricle ventricular assist device
 Written by
Megan Vermilyea,
Megan Vermilyea, MSN, CPNP-AC, is a board-certified Pediatric Acute Care Nurse Practitioner with a strong background in pediatric critical care and emergency nursing. She has worked extensively in high-acuity settings, managing complex interventions such as ECMO, VADs, CRRT, and intracardiac monitoring. At New York Presbyterian Children’s Hospital, she served as Charge Nurse and Co-Chair of the Education Committee, contributing to staff development and clinical protocol improvements.
Written by
Megan Vermilyea,
Megan Vermilyea, MSN, CPNP-AC, is a board-certified Pediatric Acute Care Nurse Practitioner with a strong background in pediatric critical care and emergency nursing. She has worked extensively in high-acuity settings, managing complex interventions such as ECMO, VADs, CRRT, and intracardiac monitoring. At New York Presbyterian Children’s Hospital, she served as Charge Nurse and Co-Chair of the Education Committee, contributing to staff development and clinical protocol improvements.
Single ventricular pathophysiology
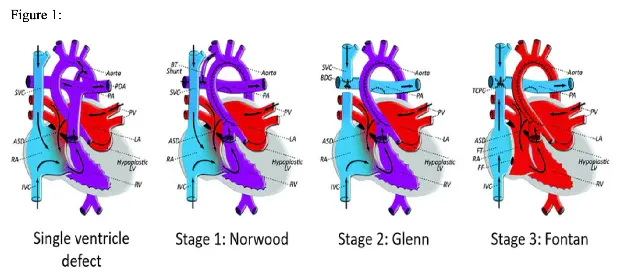
Single ventricle (SV) physiology encompasses a wide range of congenital heart defects (CHDs), including hypoplastic left heart syndrome (HLHS), hypoplastic right heart syndrome (HRHS), and various other CHDs resulting in a functional single ventricle (see Figure 1)1. Most SV patients are treated with a series of staged palliative repairs. The initial palliation, or Stage 1, consists of a Norwood operation where a “neoaorta” is created, the ductus arteriosus is closed, and a shunt is implanted to provide pulmonary blood flow. A Stage 1 palliation can also be achieved using a hybrid procedure, which consists of pulmonary artery (PA) bands with stenting of the ductus arteriosus and an atrial septectomy. Stage 1 palliations are completed in infancy, then are followed by a Stage 2 palliation (or Glenn) around age 2-3 and a Stage 3 palliation (or Fontan) around age 3-5.2 Patients may require mechanical circulatory support (MCS) at any time during these palliative surgeries due to a variety of complications including systolic or diastolic dysfunction, valvar regurgitation, or intractable arrhythmia’s.2 Extracorporeal membrane oxygenation (ECMO) has historically been used in pediatric patients due to its ability to provide both cardiac and pulmonary support, ability to support patients of any size, and relative ease of transitioning from cardiopulmonary bypass (CPB) to ECMO.3 ECMO success is affected by its’ many complications and inability to support patients long term. Mechanical circulatory support (MCS) in the form of ventricular assist devices (VADs) or ECMO can be initiated at any time during the staged palliations. MCS is often a bridge to transplant in this patient population, although it can be utilized as a bridge to recovery or bridge to destination.4 As single ventricle physiology remains the most common cardiac lesion from 6 months of age to adulthood to necessitate a heart transplant, there is a growing population of SV pediatric patients who may require MCS at some period.5 In recent years, VAD usage in SV patients has begun to grow, with the first successful VAD implantation in a SV patient reported in 2008.6
Review of VADs
Ventricular assist devices encompass a wide variety of technologies that serve to support the heart when it is in a state of low cardiac output leading to multiorgan hypoperfusion.7 These devices are either implanted surgically or placed in the cardiac catheterization lab with a goal of increasing vital organ perfusion and reducing ventricular volume and filling pressures.7 Although this technology is lifesaving, it is not without complications. The main complications of VADs include infection, thrombus formation, and risk for bleeding due to the need for systemic anticoagulation - considerations that are important when managing patients with underlying heart disease. Heightened infection risk exists as the VAD peripherally enters the skin and is situated directly inside the heart. Increased propensity for clot and fibrin formation exists due to the meeting of patients’ blood with a foreign surface (the VAD tubing).8 If a clot or fibrin does form, patients are at an increased risk of ischemic stroke if the clot is ejected from the VAD and travels to the brain. All VAD patients require systemic anticoagulation, predisposing them to coagulopathies, which commonly present as GI bleeds or hemorrhagic strokes.8 In addition, certain types of VADs, such as continuous flow (CF) devices heighten the risk of hemolysis due to the increased shear stress and narrow channels that blood travels through.8 These complications exist for all patients requiring VAD support including pediatric SV patients.
Ventricular assist devices can support patients via a continuous flow (CF) or pulsatile flow (PF) device. In adult patients it has been demonstrated that patient survival with a VAD is best achieved with a CF device.9 These devices are thought to be smaller, more reliable, and more durable.9 However, due to size constraints in pediatrics, the only currently FDA approved VAD in children is the Berlin Heart EXCOR, a pulsatile flow (PF) device.10 Pulsatile flow devices have a higher rate of device malfunction, further complicating SVAD management in pediatric patients.10
SVADs pose a unique challenge to the medical team as they attempt to maintain adequate cardiac output in the challenging single ventricle anatomy. In addition to the regular monitoring parameters and potential complications of VADs (hemolysis, coagulopathies, hypercoagulability’s, kidney injury, and infections), SVAD patients are more complex as the VAD is attempting to support solely the functional single ventricle, or the pulmonary and systemic circulations in parallel (such as in a patient post Stage 1 palliation). The attempt to balance pulmonary vs systemic circulation in an SVAD patient can inadvertently lead to increased pulmonary flows, contributing to respiratory failure and prolonging the use of invasive or noninvasive ventilatory support.10 Respiratory failure and elevated pulmonary pressures pose further complications for these patients when they undergo heart transplantation, as a newly implanted heart will often fail when it attempts to pump against high pulmonary artery (PA) pressures. Outcomes of SV patients managed with VADs has traditionally been poor, with survival to discharge rates as low as 30%.10 In comparison to SV patients’, VAD usage in a patient with a structurally normal heart generally serves to support only the left ventricle when right ventricular (RV) function is adequate. If RV function is inadequate, the RV is commonly supported with medical management. VAD management in SV patients remains challenging, and research is ongoing to discover best practice and protocols when treating these patients.
Methods
This literature review was conducted searching PubMed electronic database. Database search terms for PubMed were single ventricle AND ventricular assist device AND pediatric, pediatric SVAD, management SVAD pediatric and, univentricular VAD. Articles included were published within the last 10 years to ensure updated results. Twenty-three total articles were chosen for this literature review.
Results
VAD support at each stage of SV repair
VADs post stage 1 palliation: Stage 1 surgical palliation creates a SV anatomy with parallel systemic and pulmonary circulations. VADs are commonly implanted in this patient population due to postoperative ventricular dysfunction and inability to wean from CPB.10 Higher VAD flows (i.e. 4.0L/min/m2 in SVADs vs 2.5-3.0L/min/m2 in VADs in patients with structurally normal anatomy) are often required in this population to balance the systemic and pulmonary blood flows.11 Increased pulmonary flows can lead to pulmonary overcirculation and “pulmonary steal”, meaning that blood flows preferentially to the lungs and inadequate oxygenated blood is pumped systemically through the circulatory system. This phenomenon can lead to decreased cardiac output and end organ dysfunction. In contrast, inadequate pulmonary blood flow can lead to severe hypoxemia. Both complications will result in inadequate oxygen delivery to the tissues and systemic hypoperfusion. Newer implantation techniques, such as common atrium cannulation, can enhance cardiac decompression, thereby improving pulmonary congestion.12
VADs post stage 2 palliation: Glenn physiology refers to a single ventricle anatomy in which passive pulmonary blood flow from the upper body is achieved by a superior cavopulmonary connection (SCPC).13 (See Figure 1).1 Post bidirectional Glenn (BDG), VADs may be indicated for critical cardiogenic shock or inability to wean from continuous positive airway pressure ventilation.14 VAD management is complicated in this patient population by a continued elevation of central venous pressure (CVP) which may predispose the patient to abnormal aortopulmonary and veno-venous connections.5 In the BDG circulation, desaturated blood flows directly to the VAD through the inferior vena cava (IVC) and attempts to increase the VAD flow will therefore not improve oxygenation.14 Furthermore, BDG patients often require mechanical ventilation pre-VAD implantation. Prolonged periods of mechanical ventilation cause lung injury, and VAD implantation after prolonged lung injury does not yield good outcomes.14 In addition, pressure difference in the upper and lower venous systems for BDG patients can be further exaggerated while on VAD support, leading to development of veno-venous collaterals and hypoxia.15 Chronic hypoxia impacts end organ function, polycythemia, and anticoagulation, further complicating VAD support.15
VADs post stage 3 palliation: In Fontan physiology, all systemic venous return flows passively into the pulmonary circulation, either by an atriopulmonary connection, cavopulmonary connection, or extra-cardiac conduit.13 (See Figure 1).1 Fontan circulation can be characterized as a circulation with elevated CVP, limited effective cardiac output (CO), diastolic dysfunction, and various degrees of wasted output through systemic to pulmonary arterial collaterals.14 Although this circulation is not physiologically “normal”, many Fontan patients function in this physiologically “abnormal” state for years. VAD implantation may be required post Fontan palliation due to impaired ventricular function, or to support solely the pulmonary circulation in the case of preserved ventricular function.5 Fontan “failure” commonly occurs due to systemic venous congestion which may manifest as hepatic fibrosis, renal dysfunction, and protein-losing enteropathy (PLE), although these patents may have preserved ventricular systolic function.14 This “failure” can cause a patient to require MCS at any stage in their lives, and this support is often achieved with a VAD.
Vermilyea-MFP figure1
https://creativecommons.org/licenses/by/2.5/
Nursing considerations
Postoperative management
In the immediate post operative period, VAD settings are titrated to achieve an appropriate MAP based on patients’ age while maintaining adequate systemic oxygen delivery.10 Most patients in the immediate postoperative period require support with mechanical ventilation, inotropes, and vasodilators.10 These patients are critically ill and require one to one nursing care in a Cardiac ICU and careful monitoring and management by an interdisciplinary medical team (See Table 1). After this critical period, the goal is to wean sedatives, narcotics, inotropes and vasoactive infusions and assist the patients in getting back to a baseline (or new baseline) level of functioning.10
Anticoagulation
Patients require systemic anticoagulation during VAD support due to the inflammatory cascade that occurs as a result of bloods reaction when meeting a foreign surface. Bivalirudin (Angiomax) is often initiated in the immediate 24 hours post VAD implantation, then titrated to achieve a goal partial thromboplastin time (PTT) of 70 to 100 seconds.16 Other forms of anticoagulation, such as heparin, may be used depending on VAD type, generally with the goal of bridging to warfarin.17 Acetylsalicylic acid (aspirin) and dipyridamole (Persantine) are also initiated for their antiplatelet properties in the weeks following VAD implantation. For patients with systemic-to-pulmonary artery shunts, acetylsalicylic acid is initiated on the first night post VAD insertion.16 Similar to VAD complications in patients with structurally normal hearts, SVAD patients are at increased risk for thrombus and fibrin formation. VAD devices predispose patients to a continuous state of systemic inflammation which is often associated with hypercoagulability and can require extremely high doses of anticoagulants to achieve therapeutic levels.17 Nursing care of these patients consists of hourly monitoring of the pump and tubing to identify any new clot or fibrin formations (See Table 1). Critical care nurses are often the first to notice a new clot or fibrin formation in the VAD, and it is vital they alert the medical team.
Neurological considerations
Due to the elevated risk of fibrin or clot formation and need for continuous anticoagulation, patients receiving VAD therapy are at an increased risk for ischemic and hemorrhagic strokes, with an overall incidence of 23% in the first 2 weeks after VAD implantation.18 Neurological dysfunction remains one of the leading causes of death in patients receiving VAD therapy, and the critical care nurse must perform frequent neurological checks to monitor for any change from neurological baseline.18 In the immediate postoperative period, neurological assessments are often completed as frequently as every hour.18 These include assessment of pupils, awakening trials, presence of cough or gag, and movement of extremities (See Table 1). If new onset neurological deficits concerning for a stroke are noted, it is often recommended to obtain stat neuroimaging, generally in the form of a non-contrast computerized tomography vs a computed tomography angiography, as an MRI is contraindicated in VAD patients.18
Respiratory management
General goals of respiratory management are to extubate when able, transitioning the patient to spontaneous ventilation.19 Patients during interstage repairs often have varying degrees of underlying pulmonary pathologies. For instance, patients after Glenn palliation frequently have pulmonary pathologies such as undiagnosed pulmonary vascular occlusive disease (PVOD) which contribute to poor outcomes and make respiratory management difficult.10 Post Fontan repair, patients have persistently elevated CVPs due to a passive flow of systemic venous return into the right atrium through the cavopulmonary anastomosis. These patients also suffer from limited effective cardiac output due to prolonged use of their RV as the systemic pumping chamber. Limited cardiac output predisposes Fontan patients to pulmonary overcirculation, elevating pulmonary artery pressures. This may make future transplant more difficult, as a newly implanted heart will struggle to pump against persistently elevated pulmonary pressures. PF VAD devices pose increased respiratory management complications as the pulsatile VAD may not adequately volume unload the heart due to the continuous nature of pulmonary inflows.6 This will further contribute to pulmonary overcirculation. SVAD patients often benefit from diuretic usage to decrease preload and reduce pulmonary overcirculation. Nursing management of SVAD patients requires careful monitoring of respiratory status and fluid balance (See Table 1). In the ICU, these patients’ fluid balance is monitored hourly, and critical care nurses closely assess lung sounds, work of breathing, and trend chest x-rays to monitor for pulmonary edema or effusions.
Nutrition
Pre VAD implantation, many SV patients present with severe cardiac dysfunction that predisposes them to malnutrition.20 Once stabilized, general goals of care are to begin enteral feeding as tolerated, with the goal of progressing to full enteral feeds. Neonatal patients specifically may have difficulty with oral intake due to histories of prolonged intubations. Both nutritionists and speech and language pathologists work with SVAD patients with a goal of ensuring adequate nutrition. Since nutrition plays a strong role in recovery and return to a functional baseline, the critical care nurse must advocate for initiating enteral nutrition when feasible and collaborate within a multidisciplinary team to promote best nutritional outcomes (See Table 1).
Infection
Monitoring for infection is an important nursing implication for this patient population. Infections post VAD implantation can be grouped into non-VAD, VAD -related (including infective endocarditis or mediastinitis), or VAD-specific infections (driveline infections).4 A driveline infection (DLI) for SVAD patients can be detrimental as it can increase the risk of developing sepsis and subsequent hemodynamic instability. To reduce the risk of infection, patients receive some form of infectious prophylaxis pre- or post-transplant, generally in the form of IV antibiotics. Although VAD site care management is institution specific, at most institutions nursing coordinates dressing changes and site monitoring in conjunction with surgical or VAD teams. VAD dressings are changed once daily in the first few weeks after VAD implantation, then as infrequently as once per week. 21 Site care is performed using a variety of agents including chlorehexidine, octenidine dihydrochloride, 2% merbromin solution, hydrogen peroxide, or soap and antimicrobial sprays.21 During dressing changes, nursing monitors for signs of infection, including change in VAD site appearance or new onset redness or drainage (See Table 1). Critical care nurses also monitor for any signs of systemic infection, including fevers, leukocytosis with bandemia, or a new elevation in inflammatory markers.
Psychosocial implications
Long term VAD therapy necessitates the need for psychosocial adjustments and often leads to a decrease in psychological and emotional well-being.22 These patients often spend months (or longer) in the hospital awaiting transplant. For pediatric patients who can go home on VAD therapy, significant demands of VAD care including dressing changes, medication administration, and regular medical follow up lead to significant psychosocial stress.22 Most pediatric patients are supported with their VAD in the hospital setting while awaiting transplant or recovery. Long term hospital stays are associated with negative psychological impacts, and many SVAD patients receive psychiatric diagnoses including anxiety and depression.22 A pre-VAD implant psychosocial assessment can assist the team in identifying family strengths, weaknesses, and intervention needs.19 These findings identify the importance of a multidisciplinary team approach when caring for these patients, often consisting of psychiatrists, psychologists, consultant nurses and counselors (See Table 1). Involvement of palliative care teams can assist families in optimizing quality of life for these patients. As the critical care nurse spends the largest portion of time at the bedside caring for these patients and families, they are often the first to notice signs of depression or psychosocial concerns in patients or their family members.
Table 1: Nursing considerations in SVAD management
| Nursing considerations | VAD monitoring |
|---|---|
| Postoperative Management | Titrate VAD settings to achieve appropriate MAP Support with mechanical ventilation, inotropes, vasodilators 1:1 nursing care in Pediatric Cardiac ICU |
| Anticoagulation | Systemic anticoagulation and antiplatelets required Bivalirudin common anticoagulant used PTT goal 70-100 (varies per institution) Hourly monitoring pump and tubing for clot or fibrin formation |
| Neurological | Ischemic and hemorrhagic stroke risk Hourly neuro checks immediately post op Obtain neuroimaging if concern for stroke |
| Respiratory Management | Extubate Pulmonary pathologies related to SV physiology: pulmonary vascular occlusive disease (PVOD), veno-venous collaterals Monitor fluid balance (hourly I\&Os) Respiratory monitoring: work of breathing, lung sounds, trend chest x-rays |
| Nutrition | Cardiac dysfunction pre VAD predisposes patients to malnutrition Enteral feeds when tolerated Speech and nutrition consults |
| Infection | Infectious prophylaxis (IV antibiotics initial post op period) VAD site monitoring Monitor systemic infections |
| Psychosocial Considerations | Long waiting times for transplant lead to psychosocial distress Frequent diagnoses of anxiety and depression Psychologist and psychiatrist work with patient and families |
Discussion
Growing medical technology and surgical advances are contributing to an increased life expectancy for SV patients. Many of these patients continue to have significant complications pre- and post-surgical repair, necessitating the need for MCS. Traditionally, these patients were managed on ECMO as a bridge-to-recovery, decision, or transplant. ECMO is fraught with complications and lacks the ability to provide long term support. The ultimate treatment for SV patients is a heart transplant, but SV pediatric patients can spend months or years waiting for a heart. During this period, they may require MCS devices that provide longer term support and can return them to a more baseline level of functioning while awaiting transplant. The field of MCS has grown in recent years, and more VADs are being implanted in pediatric patients with SV physiology.6 These patients often have poorer outcomes than patients with biventricular physiology being supported with VADs, with a survival rate of around 42%.23 Critical care nurses must understand all the components that come into play when caring for these patients, including respiratory management, nutritional aspects, VAD site and infection monitoring, as well as the psychosocial implications of prolonged hospital stays and critical illness. A detailed understanding of SV physiology as well as VAD implantation techniques is essential to understanding and caring for these complex patients. In addition, the critical care nurse must be familiar with institution specific VAD protocols and potential complications. SVAD management requires a multidisciplinary team approach to treat these complex patients and their families.
Conclusions
Management and care of patients with SV physiology is challenging. These patients often develop heart failure, necessitating the need for a heart transplant.10 Although medical therapy is generally first line treatment for heart failure, long waitlist times for heart transplants in pediatric patients can necessitate the need for MCS. Although ECMO was traditionally used as a bridge to transplant in pediatric patients, the many complications of ECMO have led to VAD support becoming more common. As management and monitoring components of these patients differ from the management of MCS in patients with structurally normal hearts, it is important to understand the differing anatomies and potential complications that can occur in patients with univentricular circulations. As the field of pediatric cardiology continues to advance, there will be a growing number of SVADs implanted. Critical care nurses caring for these patients must be familiar with their institution specific protocols regarding SVADs, as well as the differing anatomies that these patients have. Critical care nurses are an integral part of caring for these patients and families, as they spend most of their time at the patient’s bedside. SVAD patients can present with subtle signs of a life-threatening complication, and the critical care nurse must have the ability and knowledge to recognize these complications and escalate the appropriate care.
Fast facts page
As technology advances and single ventricle (SV) patients continue to live longer lives, there will be a growing number of SV pediatric patients who require heart transplantation. Transplant wait times can be long, and many patients will suffer from complications that cannot be medically managed, necessitating MCS. ECMO was traditionally used to support these patients as a bridge to recovery or transplant, but its use is affected by a high percentage of complications and inability to support patients’ long term. Single ventricle ventricular assist devices (SVADs) are becoming increasingly common in pediatric cardiac centers, with the first SVAD implanted in 2008.6 A solid knowledge base of varying anatomies, VAD implantation strategies, and monitoring parameters will assist the critical care nurse in caring for this growing patient population.
- Stage I palliation: creation of a parallel pulmonary and systemic circulation through a Norwood operation consisting of a Sano or modified BT shunt, or a hybrid procedure.
- Stage II palliation (Glenn): creation of a superior cavopulmonary connection (SCPC) which provides passive blood flow to the lungs.
- Stage III palliation (Fontan): creation of a total cavopulmonary or atriopulmonary connection, providing passive venous drainage from the head and neck vessels.
- Heart failure reasons at each stage of repair vary, but causes of heart failure in this population include systolic or diastolic dysfunction, valvar regurgitation, or intractable arrhythmias.2
- Surgical implantation techniques of VADs vary based on stage of repair. The only currently approved pediatric VAD is the Berlin Heart EXCOR, a pulsatile flow (PF) device, however, other continuous flow (CF) devices are used as well.5
- Patient weight and body surface area (BSA) dictate what type of VAD can be implanted. Continuous flow devices can generally be implanted in patients with higher BSA, whereas Berlin Heart EXCORs are commonly used in smaller patients.5
- Pulsatile flow devices often have a higher rate of complications, especially thrombus formation and cerebrovascular accidents (CVAs).
- Respiratory management in this patient population is difficult, as pulmonary overcirculation can ensue. The critical care nurse must be able to appropriately monitor respiratory status and identify decompensation or signs of fluid overload.
- Nutrition: goals are to promote enteral feeding when possible and ensure adequate nutrition and hydration status. Many patients pre-VAD implantation suffer from malnutrition, and a multidisciplinary team approach can help to monitor and care for this.20
- Infection: Monitor for signs or symptoms of infection, such as laboratory abnormalities or VAD site erythema or drainage.
- Stroke: Meeting of the patients’ blood with a foreign surface predisposes these patients to a hypercoagulable state, and SVAD patients are maintained on anticoagulant and antiplatelet therapies. Frequent neurological assessments assess for acute neurological changes.
- Psychosocial implications: VAD implantation often predisposes the patient to a prolonged hospital stay, which can have negative psychosocial impacts on the patient and their family. Careful assessment of patient and family dynamics pre-VAD implantation can be vital.
References
- Trusty PM, Slesnick TC, Wei ZA, et al. Fontan surgical planning: previous accomplishments, current challenges, and future directions. J Cardiovasc Transl Res. 2018;11(2):133-144. Doi:10.1007/s12265-018-9786
- Townsend M, Jeewa A, Adachi I, et al. Ventricular assist device use in patients with single-ventricle circulation. Can J Cardiol. 2022;38(7):1086-1099. doi: 10.1016/j.cjca.2022.03.012
- Miller JR, Lancaster TS, Callahan C, et al. An overview of mechanical circulatory support in single-ventricle patients. Transl Pediatr. 2018;7(2):151-161. doi:10.21037/tp.2018.03.03
- Lorts A, Conway J, Schweiger M, et al. ISHLT consensus statement for the selection and management of pediatric and congenital heart disease patients on ventricular assist devices endorsed by the American Heart Association. J Heart Lung Transplant. 2021;40(8):709‐732. doi: 10.1016/j.healun.2021.04.015
- Burrato E, Shi WY, Ye XT, et al. Ventricular assist devices for the failing univentricular circulation. Expert Rev Med Devices. 2017;14(6):449-459. doi: 10.1080/17434440.2017.1332523
- Steffen RJ, Miletic KG, Schraufnagel DP, et al. Mechanical circulatory support in pediatrics. Expert Rev Med Devices. 2016;13(5):507-514. doi:10.1586/17434440.2016.1162710
- Kapur NK, Esposito M. Hemodynamic support with percutaneous devices in patients with heart failure. Heart Fail Clin. 2015;11(2):215-230. doi: 10.1016/j.hfc.2014.12.012
- Shah P, Tantry US, Bliden KP, et al. Bleeding and thrombosis associated with ventricular assist device therapy. J Heart Lung Transplant. 2017;36(11):1164-1173. doi: 10.1016/j.healun.2017.05.008
- Cheng A, Williamitis CA, Slaughter MS. Comparison of continuous-flow and pulsatile-flow left ventricular assist devices: is there an advantage to pulsatility? Ann Cardiothorac Surg. 2014;3(6):573-581. doi: 10.3978/j.issn.2225-319X.2014.08.24
- Merritt T, Gazit AZ, Carvajal H, et al. Evolution of ventricular assist device support strategy in children with univentricular physiology. Ann Thorac Surg. 2022;114(5):1739-1744. doi: 10.1016/j.athoracsur.2021.09.043.
- Bleiweis MS, Philip J, Stukov Y, et al. Outcomes of children supported with pulsatile paracorporeal ventricular assist device: congenital versus acquired heart disease. World J Pediat Congenit Heart Surg. 2023;14(6):708-715. doi:10.1177/21501351231181105
- Lee MM, Honjo O. Commentary: how to VAD to avoid BAD in high-risk single ventricle. J Thorac Cardiovasc Surg. 2021;162(2):414-415. doi: 10.1016/j.jtcvs.2020.09.117
- Carlo WF, Villa CR, Lal AK, et al. Ventricular assist device use in single ventricle congenital heart disease. Pediatr Transplant. 2017; 21(7). doi: 10.1111/petr.13031
- Maeda K, Nasirov T, Yarlagadda V, et al. Single ventricular assist device support for the failing Bidirectional Glenn patient. Ann Thorac Surg. 2020;110(5):1659-1666. doi: 10.1016/j.athoracsur.2019.12.088
- Chen S, Rosenthal DN, Murray J, et al. Bridge to transplant with ventricular assist device support in pediatric patients with single ventricle heart disease. ASAIO J. 2020; 66(2): 205–211. doi: 10.1097/mat.0000000000000983
- Bleiweis MS, Fudge JC, Peek GJ, et al. Ventricular assist device support in neonates and infants with a failing functionally univentricular circulation. JTCVS Tech. 2021;13:194-204. doi: 10.1016/j.xjtc.2021.09.056
- Burki S, Adachi I. Pediatric ventricular assist devices: Current challenges and future prospects. Vasc Health Risk Manag. 2017;13:177-185. doi:10.2147/vhrm.s82379
- Mills, MG, Reichhold, A, Maciorowski, et al. Stroke diagnosis protocol for children with ventricular assist devices. ASAIO Journal. 2023;69(5):199-204. doi: 10.1097/MAT.0000000000001858
- Woods RK, Ghanayem NS, Mitchell ME, et al. Mechanical circulatory support of the Fontan patient. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2017;20:20-27. doi: 10.1053/j.pcsu.2016.09.009
- Horsley M, Pathak S, Morales D, et al. Nutritional outcomes of patients with pediatric and congenital heart disease requiring ventricular assist devices. JPEN J Parenter Enteral Nutr. 2022;46(7):1553-1558. doi:10.1002/jpen.2351
- Özdemir Z, Şenol Çelik S. Wound care of the driveline exit site in patients with a ventricular assist device: a systematic review. Turk Gogus Kalp Damar Cerrahisi Derg. 2018;26(2):328-335. doi: 10.5606/tgkdc.dergisi.2018.14982
- Rea KE, McCormick AM, Lim HM, et al. Psychosocial outcomes in pediatric patients with ventricular assist devices and their families: a systematic review. Pediatr Transplant. 2021;25(4):e14001. doi:10.1111/petr.14001
- Miller JR, Eghtesady P. Ventricular assist device use in congenital heart disease with a comparison to heart transplant. J Comp Eff Res. 2014;3(5):533-546. doi:10.2217/cer.14.42
List of figure captions
Figure 1
In this image of a hypoplastic left heart, deoxygenated blood returns to the right atrium (RA), then is pumped through the tricuspid valve, out the pulmonary artery and to the lungs. A patent foramen ovale (between the atrium) provides mixing of oxygenated and deoxygenated blood, and a patent ductus arteriosus provides systemic blood flow.
In the Stage 1 depiction, a BT shunt has been created (innominate to right pulmonary artery) to provide pulmonary blood flow, and a “neoaorta” has been created to provide systemic blood flow. Stage 2 depicts a SCPA from the SVC to RPA, allowing blood to flow passively to the lungs from the head and neck vessels, then return to the left atrium (LA) and be pumped out through the neoaorta. Stage 3 depicts a patient with TCPA (total cavopulmonary anastomosis). The IVC has been re-anastomosed to the SVC, passively draining blood to the lungs. Oxygenated blood is then returned to the LA, travels through the foramen ovale and is pumped systemically through a neoaorta.1
Did you get it?
- To prevent clot formation caused by the VAD's foreign surface
- To increase the flow rate through the VAD
- To treat existing ischemic strokes
- To reduce the need for respiratory support
- Higher rates of thrombus formation and CVAs
- Inability to support systemic circulation
- Hemolysis due to narrow flow channels
- Difficulty implanting the device due to its small size
- Pulmonary overcirculation due to unbalanced flow
- Pulmonary artery stenosis from high VAD pressure
- Systemic hypertension caused by VAD malfunction
Stay on track!
Would you like a reminder when your ACLS certification expires, plus study tips?
How we reviewed this article
Our experts continually monitor the medical science space, and we update our articles when new information becomes available.
-
Current versionMail the authorEmail
- Aug 05, 2025
-
Written by:
 Megan Vermilyea
Megan VermilyeaMegan Vermilyea, MSN, CPNP-AC, is a board-certified Pediatric Acute Care Nurse Practitioner with a strong background in pediatric critical care and emergency nursing. She has worked extensively in high-acuity settings, managing complex interventions such as ECMO, VADs, CRRT, and intracardiac monitoring. At New York Presbyterian Children’s Hospital, she served as Charge Nurse and Co-Chair of the Education Committee, contributing to staff development and clinical protocol improvements.